CiFlu. RECOMBINANT INFLUENZA VACCINE
CiFlu.
A sub-unit vaccine for the treatment of seasonal and pandemic flu.
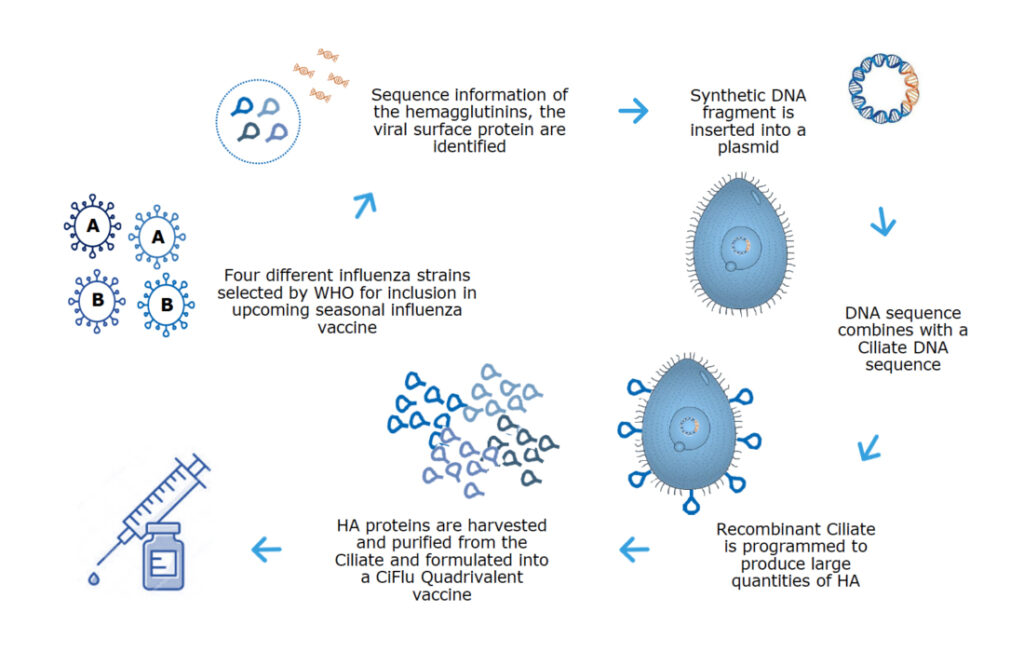
With the genetic information of the antigen, Tetrahymena cells are reprogrammed to produce the antigen of the flu virus. This antigen is purified and further processed into a protein-based vaccine.
The vaccine is a quadrivalent protein subunit vaccine produced using a virus-free recombinant DNA platform. It produces an exact genetic match to the target antigen hemagglutinin (HA) of both A and B strains of influenza viruses.
CiFlu has shown high and complete inhibitory immunogenicity in vivo, which means complete protection against the virus. Due to its high efficacy, the CiFlu subunit vaccine can be used, if desired, without adjuvants and is compatible with any desired adjuvant.
High safety and efficacy
Fewer side effects expected
No egg components
Quadrivalent to protect against four different influenza viruses
No changes in hemagglutinin antigen sequence that could lead to reduced vaccine efficacy
Advantages of recombinant DNA technology
Rapid scalability in the event of a pandemic compared to the use of conventional production facilities
No embryonated chicken eggs required
No biocontainment facilities required
No virus involved
No solvent inactivation or organic extraction procedures required
Proven in vivo efficacy in non-human primates
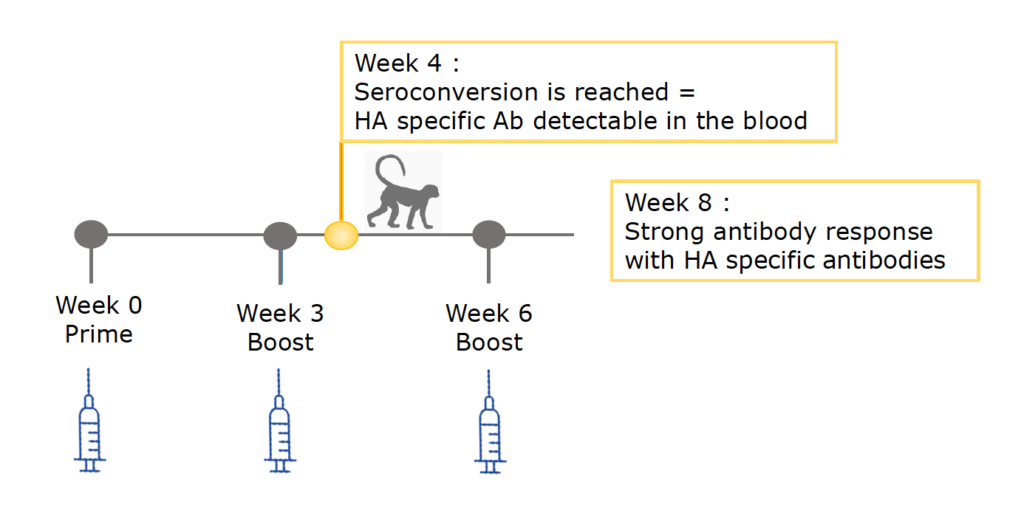
Vaccination of macaques with HA (hemagglutinin) produced in ciliates induces a strong antibody response.
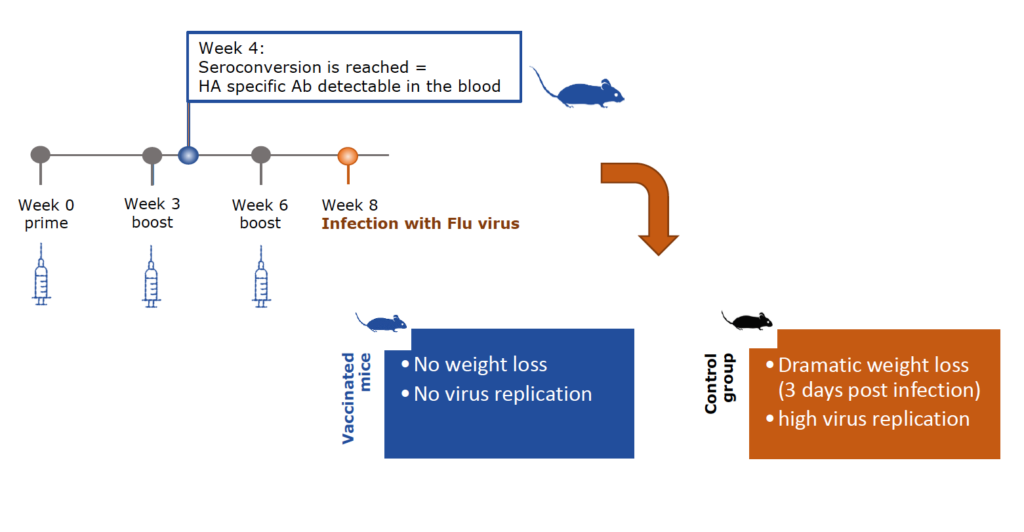
Vaccination with CiFlu protects mice from influenza infection

