Ciliate Lipase.
THERAPEUTIC ENZYMES.
Ciliate Lipase.
A new active ingredient for
Exocrine Pancreatic Insufficiency (EPI).
A new active ingredient for Exocrine Pancreatic Insufficiency (EPI). Exocrine pancreatic insufficiency (EPI) is a serious and life-threatening disease. It is characterised by a deficiency of pancreatic enzymes, resulting in the inability to digest food properly. This primarily affects human lipase, an enzyme that breaks down fat, proteases, enzymes that break down proteins, and amylases, enzymes that break down sugars. The lack of these enzymes can be responsible for indigestion, steatorrhea, the urge to defecate and weight loss.
EPI is caused by severe disease of the human pancreas for a variety of reasons, such as cystic fibrosis, chronic pancreatitis, pancreatic cancer and many more. The standard treatment for EPI is the oral administration of pancreatic enzymes. All currently approved enzyme preparations are derived from pig pancreases, which are obtained from slaughterhouse waste.
Pig enzyme replacement therapy vs. Ciliate lipase
→ 3 x 300 mg pancreas powder
→ Capsule size 0
→ High number of capsules (~ 4 to 5 per meal)
→ Low acceptance and poor dosage compliance, especially in case of young sufferers
→ 1x 150 mg Ciliate lipase
→ Capsule size 4
→ Low number of capsules (1 per meal)
→ Improved comfort and high potential to improve patient acceptance
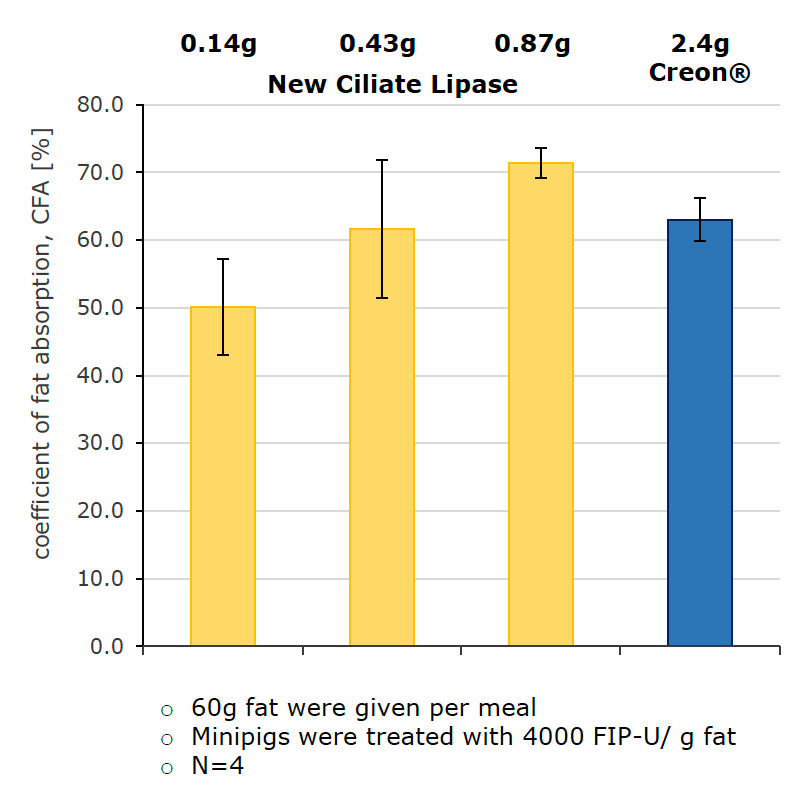
Proven in vivo efficacy in EPI mini-pigs
Oral administration of Ciliate Lipase resulted in CFA (fat absorption units) values that were comparable to creon.
Further doubling of the amount of lipase resulted in a higher CFA value, indicating that the maximum effect is not achieved with the given maximum dose.

Our production process
- Cell banking
- Fermentation
- Purification through crystallisation
- Formulation

